
New guidance jointly developed by the NHS Confederation and the Association of the British Pharmaceutical Industry. View an overview of the guidance.

Foreword
Cross-sector collaborations between NHS organisations and the research-based pharmaceutical industry have a strong track record of delivering benefits for patients, the NHS and industry – the so-called ‘triple win’. You can explore the wealth of health system efficiencies and patient benefits gained from these collaborations in the ABPI’ s library of cross-sector initiatives.
Despite these successes, we believe the potential of cross-sector partnerships to accelerate health system transformation is still to be realised. Now that England has formally integrated health and care across primary, secondary and social care, it should be possible to make a step change in the scale and ambition of cross-sector collaboration and demonstrate measurable correlations between project interventions and patient outcomes.
The NHS Confederation and ABPI have now been working together with industry and system leaders for seven years to understand and unblock the barriers to making the potential of partnership a reality – exploring issues such as culture, trust and the sheer challenge of stepping outside health system operational norms.
In doing so, we have heard directly what NHS leaders and industry need to work more easily together:
- practical resources and sources of assurance to enable scoping, implementation and reporting across the lifecycle of partnership programmes
- greater understanding by NHS leaders of the regulations governing industry collaboration.
This new guidance aims to meet those needs as the next step in our work together – providing a practical, step-by-step guide to help NHS and industry develop, contract, implement, measure partnerships more easily, and deliver the benefits more rapidly.
Key to this new guidance is the introduction of recommended frameworks, endorsed by both our organisations, that can be used by NHS organisations at all levels – trusts, practices, networks and integrated care systems – to make each stage of partnering straightforward, both for the initial project and for scaling to other locations. We are indebted to the multiple NHS and industry leaders who gave their time to help us design this guidance and hope that it ushers in an uplift in the scale and ambition of collaboration between our sectors to transform the NHS and improve patient care.
Matthew Taylor, Chief Executive, NHS Confederation
Dr Richard Torbett, Chief Executive, ABPI
About this guidance
This guide provides a practical, step-by-step to help NHS and industry develop, contract, implement, measure and deliver benefits more rapidly. It contains template forms, recommended frameworks, and handy checklists and prompts to support you.
Who this guidance is for
This document is aimed at local NHS organisations in England, industry leaders and those leading on the partnership and transformation agenda within their organisation or system. Use it to:
- bring stakeholders and partners together to assess priorities for NHS-industry partnership working
- design and implement partnership projects aligned to strategic objectives and informed by the guidance resources
- strengthen assurance and nurture the culture of effective partnership working
- scale existing partnerships across care settings.
Guidance at a glance
Click on the image to download a PDF overview of the guidance:

What are NHS-industry partnerships?
NHS-industry partnerships allow local NHS organisations and the pharmaceutical industry to collaborate for patients’ benefit.
Since joint working was established in 2008, the Department of Health and Social Care (DHSC) has acknowledged the value of external expertise in helping NHS organisations overcome challenges. This includes providing additional skills and resources to achieve patient benefits beyond what NHS organisations could do alone. These partnerships not only offer innovative treatments to enhance patients’ outcomes, but also bring industry skills and expertise to support project management and the efficient delivery of healthcare services.
Partnerships can be formed between a single NHS organisation and a single pharmaceutical company, or multiples of either. In general, there is a trade-off between the advantages of greater scale in working with multiple partners, such as provider collaboratives in England, and the added complexity of gaining agreement across multiple organisations. While patient organisations cannot directly be included in collaborative working arrangements, occasionally they may be contracted to deliver a service to support an element of such collaborative working.
The benefits of working together
Working across sectors can accelerate improvements in patient care at an organisation, population or system level. Often cross-sector projects will pilot innovative models of care that can be replicated and scaled up. Pharmaceutical companies can bring much-needed expertise, skills and resources to complement the expertise of healthcare organisations and patient organisations, such as:
- data evaluation, health, economic and project management expertise
- medical writing, business management, marketing and communications skills
- process redesign
- educational resources
This triple win – benefiting patients, healthcare organisations including the NHS, and pharmaceutical companies – is more important than ever in the context of the challenges facing healthcare organisations. The online ABPI-NHS case study library brings together over 180 examples of projects set up, delivered and resourced by NHS and pharmaceutical industry partners. Unlike the provision of grants and sponsorships by pharmaceutical companies, or simple provision of services such as homecare, or patient-support programs, NHS-industry partnerships represent a true pooling of skills, experience, and resources from all parties involved.
Collaborative working and joint working NHS-industry partnerships, which are the focus of this guidance, and described in more detail below, are a specific type of activity which is defined and described in the ABPI Code of Practice. These requirements including those pertaining to set-up and approval of the collaborative or joint working partnership, as well as specific transparency stipulations form key guardrails around such partnerships. These are described in more detail, below.
Types of NHS-industry partnership
There are two types of NHS-industry partnership: collaborative working and joint working projects. These partnerships involve cooperation between industry and local NHS organisations across primary, secondary and system-level healthcare settings.
Collaborative working is generally between one or more pharmaceutical companies, healthcare organisations and possibly other organisations. It must have, and be able to demonstrate, the pooling of skills, experience and / or resources from all parties involved. There must be a shared commitment to successful delivery from everyone involved and each organisation must make a significant contribution. In the case of NHS organisations, this contribution does not have to be financial. It can involve the sharing of support in the form of skills and experience to deliver projects successfully.
Joint working projects are a specific type of NHS-industry collaborative working, rather than a generic term for all cross-sector collaboration. They must be patient-centred and always benefit patients directly, which gives them a narrower focus than collaborative working.
Collaborative and joint-working approaches: a comparison
| Collaborative-working projects | Joint-working projects |
|---|---|
| Are for the benefit of patients and/or the healthcare organisation, including the NHS. | Must always be for the benefit of patients directly and must include the NHS as a party. |
| Enhance patient care or be for the benefit of patients, or alternatively benefit the NHS and, as a minimum, maintain patient care. | |
| May not constitute a grant/donation (see Clause 23 of ABPI Code of Practice for further information on Donations and Grants). | |
| May provide benefits to the company or companies involved. | |
Outcomes must be defined in such a way that they can be measured or tracked, so that at any time during the collaboration all parties are aware of:
| |
| Must be carried out in an open and transparent way, with a certified summary of the project agreement publicly available before it begins. | |
| Must respect clinical independence. | |
| Must be prospective – not relating to a project that has already begun. | |
| Must have the value to the healthcare organisation publicly disclosed annually on the Disclosure UK database and, if relevant, the contracted service value to the patient organisation published on the industry partner’s website. | |
| Must not constitute an inducement to health professionals or other relevant decision-makers to prescribe, supply, recommend, buy or sell a medicine. | |
| Must ensure that the rights and legitimate interests of all parties are continuously observed throughout, including considerations related to data security, the protection of confidentiality and privacy, and anti-bribery compliance. | |
| Must not promote a prescription-only medicine to any member of the public. | |
As noted in the table, one of the key benefits of collaborative working is the ‘triple-win’ - benefiting patients, healthcare organisations including the NHS, and pharmaceutical companies. Benefits for pharmaceutical companies in embarking upon collaborative working can be myriad, from gaining experience in partnering with an NHS organisation, to an increase in patient identification and prescribing in accordance with national and local guidelines. Most importantly however, and in accordance with Clause 20 of the ABPI Code of Practice, such benefits must not constitute an inducement to health professional or other relevant decision makers to prescribe, supply, recommend, buy or sell a medicine. A key safeguard here is the requirement in the ABPI Code of Practice to have a have a summary of the collaborative working agreement publicly available before arrangements are implemented.
It is also worth noting that in embarking upon collaborative working with NHS organisations, companies will have no direct contact with patients, or with identifiable patient level data.
The ABPI Code of Practice
The 2024 ABPI Code of Practice (see Appendix 1 for further details) exists to regulate the promotion of prescription medicines to UK health professionals, industry interactions with health professionals, and the provision of information about prescription-only medicines to the public.
It is administered by the Prescription Medicines Code of Practice Authority (PMCPA) and is the cornerstone of the UK system of industry self-regulation. All NHS-industry partnerships are bound by the ABPI Code of Practice.
“NHS-industry partnerships are bound by the code, which serves as a guardrail by which industry is regulated to ensure that throughout all collaborations, patient safety is maintained, in a professional, ethical and transparent manner to ensure the appropriate provision of high-quality care.” Dr Amit Aggarwal, Executive Director, Medical Affairs and Strategic Partnerships, ABPI
Achieving impact with partners
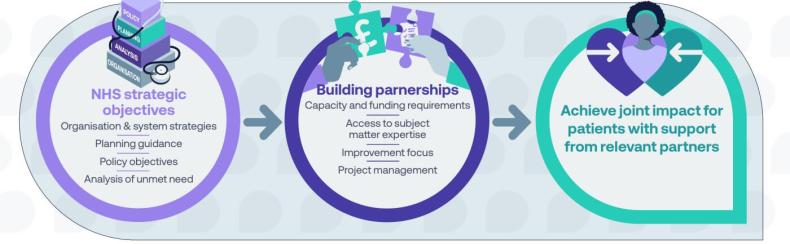
Supported by NHS-industry partnership guidance and ABPI Code of Practice.
Key stages of the project lifecycle
The figure below outlines the stages of a partnership, from planning to delivery and monitoring:
Stage 1: Project identification and scoping
- Where can partnership projects originate?
- Scoping decision guide
Stage 2: Project setup and governance structures
- Project setup and governance structures
- Stakeholder engagement
Stage 3: Project implementation and outcomes reporting
- Project delivery and evaluation
- Reporting on the project outcomes
Stage 1: Project identification and scoping
“NHS leaders are working hard to find ways to deliver high-quality services, while also helping people to stay healthier longer and seeking to reduce inequalities. Opportunities presented by effective NHS-industry partnership working have a valuable contribution to make to these efforts. This joint NHS Confederation and ABPI guidance is designed to enable teams to collaborate with confidence in the interest of the local communities that they serve.” Sarah Walter, Director, NHS Confederation’s ICS Network
Where can partnership projects originate?
Primary care
Primary care or primary care network (PCN) partnerships often arise from existing relationships with industry providers. At other times, this will take place through proactive work by PCNs, federations and individual practices interested in finding partners to support specific projects. Industry partners will also often research and directly scope out primary care providers where projects can directly support their existing clinical priorities.
Secondary care
Across secondary care settings, partnership working is well established and, as such, clearer routes exist when identifying projects. Often agreed avenues for exploring partnerships are in place, such as via relationships between industry and trusts, as well dedicated commercial services teams which will issue expressions of interest regarding partnership objectives and opportunities. This is further enhanced by the wealth of existing secondary care networks, which enable a greater spread of successful projects from one locality to another.
Integrated care system/ place level
Partnership working with integrated care boards (ICBs) in England can be more challenging to establish. It is important therefore that any prospective partnership is underpinned by mission-oriented priorities that directly support the system’s national obligations and local objectives. Early-stage discussions about entering a collaborative or joint-working arrangement with board staff should include relevant clinical leads and executive leads, including the chief pharmacist as appropriate.
If proposals are deemed suitable to explore further, it is advised that the designated NHS and industry leads should provide initial details on the scope and aims to relevant board-level working groups, such as the ICB Integrated Medicines Optimisation Committee (IMOC) or equivalent medicines committee, for their consideration. The 15 health innovation networks (HINs) in England that operate across ICBs can also serve as an important conduit by which to streamline processes of setting up system-level partnerships aligned to system priorities.
Key resource: Scoping decision guide
At the start of a partnership, NHS and industry organisations need to identify unmet needs to support clinical outcomes. Industry partners often align their capabilities with NHS goals or issues identified through health data analysis.
This process includes community engagement and patient feedback to identify opportunities for improvement. Colleagues should use these questions to explore and develop the scope of a project, to ensure the aims are clear and the entire lifecycle of the project has been considered.
| Questions to consider during the scoping of a project | Questions to consider if the project has a therapy/medicine focus |
|---|---|
| What is the unmet need the project is seeking to address? | Which clinical pathway(s) require service redesign to improve patient care and/or system improvement? |
| What is the scale of the problem? | |
| What is the evidence to back this up? | Which companies have expertise in this area? |
| What is the priority improvement area, and can this realistically be addressed within the resources and duration of the project? | |
| What are the patient or system benefits of addressing this need? | Are there other organisations that would be relevant to engage in the project? For example, at an ICS level, can HINs play a convening and facilitation role for the partnership? |
| What would be the impact on patient care or the system if this need is not addressed? | |
| Are other internal stakeholders supportive of addressing the need and the feasibility of doing so? | |
| Which NHS plan and/or local improvement plan goal is the challenge aligned to? | |
| What interventions are required and in what timescale? | How are partners considering the impact of health inequalities and equality of healthcare access? |
| What is the likely impact on the clinical and non-clinical workforce? For example, will this project involve complex pathway redesign? | |
| Are prospective partners clear on existing operational pressures, and their potential impact on the project? | |
| What related challenges need to be addressed in other parts of the system for the project to succeed? | |
| What does success look like? How will it be measured? When and by whom? | |
| Is the project intended to demonstrate a sustainable solution? If so, what outcomes will be necessary to ensure a successful business case? | |
| Is there capacity to release the necessary internal team members to participate in the project? |
Key resource: Checklist for determining if a project is collaborative working, joint working or neither
To help identify if a project is a collaborative or joint-working project, an assessment of the project scope and aims should be undertaken, which can be supported by completing the checklist.
If the answer to any red questions on the checklist is ‘No’, the project is not a collaborative or joint working arrangement, and will need to be modified before proceeding. If changes cannot be made, prospective partners should consider an alternative approach such as a research collaboration or a donation/grant, as described in Clause 23 of the 2024 ABPI Code of Practice. If the answer to any amber questions is ‘No’, this signals an issue or risk that should be addressed to encourage successful and timely project delivery.
Key resource: Project Concept Framework
The key recommended documentation for stage 1 is a Project Concept Framework, outlining the project’s purpose, objectives, resource impact and timelines. It must be approved by all relevant parties to support progression to the project setup stage.
Case study 1
View a case study demonstrating the step-by-step implementation of a project in a primary care setting.
LOGIC – hoListic prOactive manaGement in chronic obstructive pulmonary disease (COPD): AstraZeneca UK Ltd and Hartlepool Health Primary Care Network (PCN) Joint Working Arrangement
The project objectives
- There was an unmet clinical need in Hartlepool Health PCN with a higher than national COPD prevalence and it was ranked third highest for hospital admissions in England.
- The project therefore looked to fund time for COPD nurses to conduct high-quality COPD reviews and provide clinical supervision to upskill nurses in the PCN. The project also looked to:
- improve adherence from multiple inhaler triple therapy to single inhaler triple therapy
- identify and review patients with cardio-pulmonary risk factors
- reduce healthcare resource utilisation by optimising the management of COPD patients.
How was the project identified
- The project was identified through COPD heat maps, using prevalence, admissions, and prescribing data which showed outcomes were worse in this locality than elsewhere.
- AstraZeneca had a relationship with a respiratory nurse regarding education and information, and this helped facilitate the partnership.
- The clinical pharmacist, in line with national guidance, was motivated to reduce the overuse of oral corticosteroids.
- The local medicines optimisation pharmacist wanted to reduce the number of multiple inhalers being used, in line with local guidance, to a fixed dose triple which their guidance positioned would be better environmentally and financially.
Guidance used
- ABPI guidance on joint working, which meant all parties were committed to achieving the outcomes. The principles of joint working underpinned the contract.
- There was an explanation of what joint working meant from a business-to-business contractual relationship.
Risk management
- Through regular, fortnightly meetings which reported on the progress of key performance indicators (KPIs) and timely responses on all sides to solve challenges along the way.
- Development of a robust exit strategy.
How was the project monitored and reported?
- Fortnightly meetings against KPI’s of the joint working agreement.
- Through the NHS project manager and clinical pharmacist.
- Having a clear Responsible, Accountable, Consulted, and Informed (RACI) chart for all stakeholders involved.
- Documenting each deliverable in a way that maintained private confidentiality as well as maintaining the standards set out in the ABPI Code of Practice.
Outputs
- Improvements to COPD outcomes and a greater prevention of hospital admissions.
- The care team's wellbeing was enhanced through supporting the upskilling of workforces through legacy mentoring.
- Improved patient experience by supporting patients with education about their disease and through social prescribing.
- Reductions in medicine wastage and sustainability resulted in cost savings, particularly through the transitioning of patients from using multiple inhaled therapies to single-inhaled therapies.
Stage 2: Project setup and governance structures
Project setup and governance structures
At this stage, a project team or steering committee is formed, including all involved parties and active participants. This team will guide and manage the project, being responsible for its success and operating within the agreed limits.
The members of this project team may differ depending on the care setting. Examples
of stakeholders who might be required to participate in this team can be found below. The team should be ‘right sized’ to be both effective and inclusive.
All project team or steering committee members must declare any conflicts of interest. As such, they should follow NHS England’s Conflicts of Interest Guidance, which was co-developed with the ABPI.
Those with a conflict should not vote on related matters. These declarations should
be recorded in the meeting minutes. Either party can object to someone’s involvement
due to a conflict of interest.
The project team should agree on a project methodology. PRINCE2 (Projects In Controlled Environments) is perhaps the best-known and most widely used by the NHS, but the methodology may vary depending on the complexity of the project. It should also agree on a regular meeting schedule to make initial decisions, keep the project on track and manage any issues. Meetings should be action-oriented, with clear agendas, decision points and minutes recorded.
When considering project resourcing, all parties must commit to detailing the resources they will contribute to the project, which could include finances, skills or experience. These contributions should go beyond normal day-to-day roles, like funding additional staff or clinics. Assigning a monetary value to NHS resources is challenging, so contributions should be comparable and proportionate. If the collaboration aims to address healthcare organisation constraints, precautions must be taken when using pharmaceutical funding to retain staff. Any staff paid through industry funding should operate under NHS control, with clear employment law compliance. Exit strategies for industry-funded posts should be outlined in project documents.
Key resource: Project team / steering committee members (non-exhaustive)
Who should be in a project team/project steering committee? (non-exhaustive)
| Care setting (options depending on local context) | Key stakeholders |
|---|---|
Primary care
| Industry
|
Secondary care
| Industry
|
System-level care
| Industry
|
Due to the non-promotional nature of collaborative working projects, none of these roles should be from the sales function within companies.
Key resource: Steps to build trust
Trust is fundamental to any partnership, especially during the early scoping phase. All parties should ensure that they work towards engendering trust which can be supported through the following steps:
Process and organisation
- Developing a shared vision together, aligned to priorities to improve capacity, capability and outcomes
- Transparent decision-making
- Joint ownership of decisions and collective responsibility for direction, activities and outcomes
Behaviour
- Recognition of the value of each party’s contribution
- Maintaining deadlines and actions
- Sharing knowledge
- Demonstrating the ability to be flexible and adaptable
- Ability to compromise
- Acknowledgement of cultural difference
Continuity
- Not moving goalposts (such as pulling budgets, changing priorities)
- Minimal personnel changes or at least good practice in transition
Outcomes
- Clear recording and reporting of outcomes
- Agreed plans for upskilling and knowledge transfer
- After Action Review or similar feedback and learning processes
Key resource: Project Initiation Document (PID)
Once the necessary criteria have been fulfilled, the project team should develop and approve a project initiation document (PID) to ensure a shared understanding of the project’s outcomes, its governance framework and to provide a clear exit strategy that details the overall responsibility of each party if the project needs to be terminated.
The PID is a key document that sets out requirements ahead of project implementation and the agreed copy should be kept on record for both parties’ reference. Confidentiality of patient information must be maintained in all partnerships, as outlined in the PID. This includes respecting the confidentiality of project-related information and not sharing it beyond the project’s scope. The PID should be collaboratively created by relevant individuals from all partnering organisations.
It is important to note that PIDs will, at times, need to be updated following the initiation of the project. This can be addressed via a project amendment form.
The PID must be certified by the industry partner and be approved by both the project team also the relevant governance committee(s).
Access the Project Initiation Document.
Establishing a robust governance framework
When entering into a partnership initiative, industry and NHS organisations need to ensure they are working within a robust governance framework to ensure the project aligns with their organisations’ goals and legal processes.
This involves establishing a governance committee to oversee the project. The governance committee will also review the principles of the project against the collaborative and joint-working checklist criteria and ensure that the project has been reviewed by each participating organisation’s management and experts.
Within pharmaceutical companies, governance expertise will be provided by legal, medical, compliance and healthcare engagement functions. Within NHS organisations, governance will usually be provided by existing governance committee or Internal Review Committee (IRC). For collaborative projects, one must find stakeholders with the authority to approve the project.
Examples of key individuals who can form part of a governance committee are presented below:
Key resource: Potential governance committee members across care settings (non-exhaustive)
- Named executive senior responsible officer from each party to the partnership
- Relevant clinical lead
- Programme management and support
- Insight and intelligence teams
- Pharmaceutical legal director
- Pharmaceutical medical / compliance director
Stakeholder engagement
Building confidence in the project with stakeholders, both internal and external, is vital to avoid misunderstandings and ensure transparency.
This includes clarifying aligned interests and disclosing benefits to industry transparently. Clear communication between partners is essential to refine project objectives, manage expectations and confirm inputs from each organisation. At this stage, the project team should develop a stakeholder map, communications plan and data collection plan if not already done in the PID. Realistic timescales should be set, with the first three steps taking four to six months, including scoping, development and approval of governance arrangements and legal framework.
It is a helpful exercise to divide stakeholders between internal and external stakeholders who:
- will ultimately be impacted by the outcome
- whose opinions could facilitate or prevent success.
- should be involved in the project
- are not directly involved in the project, but whose views could influence the outcome and who should be kept informed throughout the entire project lifecycle
Key resource: Examples of stakeholder groups to engage across care settings (non-exhaustive)
| Primary care | GPs, pharmacists, patients and residents, local Healthwatch and voluntary and community sector organisations |
|---|---|
| Secondary care | Chair of Trust Quality Committee, Chair of Medicines Optimisation Group, patients and residents, local Healthwatch and VCSE organisations |
| System-level care | Chair of ICB Quality Committee, patients and residents, local Healthwatch and VCSE organisations |
| Industry | Company decision makers, local representatives, market access teams, project managers |
| Others | NHS England, Department of Health and Social Care, patient groups |
Patient engagement
NHS and industry partners should give due consideration to the impact on patients, and if appropriate, gain feedback from patients or patient groups. Ways to incorporate patients’ experiences can include:
- patient stories of their experiences
- mapping the key pathways of service with patients and staff working in coordination
- considering community representation so that plans being developed represent diversity and the needs of different groups impacted, and ensuring inequalities are considered
- recording a patient’s experience of a service and asking for their views following the completion of the project for inclusion in the project outcomes.
Recommended Collaborative/Joint Working Agreement Framework
Once the project has been approved in principle by all relevant parties, the project team must work with its organisational legal experts to draft and sign a Collaborative/Joint Working Agreement. The agreement is a legal contract that will include key information about the project and plans, drawn directly from the PID. It must be entered into with legal, corporate entities and not with any individual in primary, secondary and system-level settings. The agreement must ensure that any confidential, competitive, or personal data are protected by strong contractual provisions. It should include those listed in this document.
Please note: Any collaborative or joint-working agreements must be entered into with legal, corporate entities and not with any individual member of staff across primary, secondary, and system-level settings.
The executive summary
The last stage in the project setup is the publication of an executive summary, which will largely draw from the content issued in the PID.
As outlined in the ABPI Code of Practice, an executive summary of the project rationale, period and objectives must be published on the industry partners’ website once the project has commenced. It is also advisable that relevant NHS partners do the same). The project should not commence until the executive summary has been published on the relevant industry partner(s)’ website. A recommended executive summary framework is shown below:
Key resource: Recommended Collaborative / Joint Working Project Executive Summary Framework
Case study 2
View a case study demonstrating the step-by-step implementation of a project in a secondary care setting.
Review of the current chemotherapy service pathway and provision for systematic anti-cancer therapies (SACT) across Lancashire and South Cumbria Cancer Alliance
The project objectives
- To carry out a service evaluation to see how patients accessed the service; the time they were waiting at each stage of the pathway, especially in terms of receiving treatment; and at which NHS organisation they are being seen against where they lived.
- To ensure equity of pathway access, provision, and experience for patients in Lancashire and South Cumbria through reductions in unwarranted variations in care.
- To explore potential improvements to the pathway to improve the experience and care received by the patient.
- To explore potential improvements to the pathway to improve system capacity and reduce the amount of time patients spent in a hospital to receive their treatment.
- To develop an options appraisal for trust partners to ensure that the service is fit for new and future demands.
How was the project identified?
- Sanofi was active in the cancer therapy area with a specific interest in skin cancer. Patient treatment inefficiencies and excessive travel were issues for the trust in the delivery of chemotherapies. Through this, a common area of interest was found.
- The project was developed following two years of interactions with the team at Preston and the Sanofi market access team. This ensured that there was a rapport built, as well as new relationships leading to key decision-makers becoming involved. This included the clinical lead, service manager, lead chemotherapy nurse, and oncology pharmacist.
- Through these relationships, an idea emerged to overcome the issues of pooling resources, utilising Sanofi project management skills, and use the findings to build business cases for change.
- The trust did not have the time and resources to overcome these on its own.
Guidance used
- The ABPI Collaborative Working Project checklist was used as a basis by which to answer if the project was indeed a collaborative project.
- Additionally, Sanofi’s Internal Collaborative Working Standard Operating Procedure, written using the ABPI code as the framework, was used as a key resource. Including a ‘collaborative working on a page’, this document helped explain to partners the process and what documentation needed to be completed at each stage.
- A governance committee was established to help oversee the development of the project documentation, specifically approval of the concept and project initiation document. This governance committee has oversight of all projects.
Risk management
- A clear exit strategy was included in the project initiation document and contract, stipulating that if either party was unhappy at the end any stage the project could be terminated.
- Multiple team members were involved in the process, ensuring buy-in, and ensuring that if the main partner left for any reason, the project would continue. As a result, although the clinical lead did take a sabbatical during the project, this did not stop the project from continuing.
How was the project monitored and reported?
- The project was monitored through:
- regular group meetings with clear agendas
- a project plan, which included objectives and a clear responsibility for the task
- relevant actions were monitored at every meeting.
- Data – all anonymised for analysis.
- Surveys – staff and patient surveys, service analysis.
- Project executive summary and end report, written and published on both Sanofi Corporate website and the trust website.
Outputs
- Outputs from the project included an options appraisal for suggested changes to be made by the trust.
- Mapping of the patient pathway and identification of steps where efficiencies could be made:
- Telephone immuno-oncology service – better utilisation of this service.
- Chair time is taken up by inappropriate treatments.
- Pre-assessment – extra resource required to ensure chair time didn’t have to be cancelled.
- Funding secured from the Cancer Alliance to resource extra posts in the department.
- Mapping of the patient pathway and identification of steps where efficiencies could be made:
Stage 3: Project implementation and outcomes reporting
“Building and managing successful partnerships is difficult, and healthcare teams often face complex challenges when attempting to innovate or work in new ways. The guidance set out by NHS Confederation and ABPI helps to show the clear steps that can support effective collaboration.” - Clair Huckerby, Chief Pharmacist, Consultant Pharmacist Primary Care, Our Health Partnership
Project delivery and evaluation
Once the collaborative agreement is signed and published on the industry partners’ website, the project officially starts.
To monitor project progress effectively, partners should refer to the outcomes outlined in the PID and executive summary. Collaborative and joint-working projects are not set up as clinical trials or real-world evidence generating trials, and as such the project metrics need to be realistic and based upon the objectives of the project and intended purpose thereafter, ie business case or scalable solution. Examples of relevant metrics include clinical impact, delivery, service effects and economic impact. After project completion, outcomes will be measured and documented, with stakeholders and the project team evaluating learnings from the project.
Key resource: Project monitoring guidance
| Regular monitoring is essential to ensure that: |
|
| Effective monitoring should also comprise: |
|
If an overrun or delay looks likely, the project team should agree on mitigating actions and amend plans as necessary. This can be recorded in a letter of amendment or extension, known as a variation agreement, which the pharmaceutical company’s legal team can draft on behalf of the project team.
If significant changes to the project occur during implementation, the following form should be completed and agreed by the project team and the governance committee in order to keep a record of the project aims as part of good governance:
Key resource: Recommended Project Amendment Framework
Reporting on the project outcomes
It is important to recognise that successful organisations will learn from
their experiences of partnerships.
Learning is more beneficial when it is preserved beyond the end of the project in the outcome report. As well as evaluating the outcome of the project, it is useful to assess how successful or unsuccessful the operation of the project has been so that lessons can be learned and can be usefully applied in the design and running of other projects.
As stated in the ABPI Code of Practice, all parties should publish outcomes promptly, within six months. Local NHS organisations are encouraged to do the same. To promote and expand successful collaborations in healthcare, these outcome reports should be shared with ABPI for their NHS-Industry Partnership Case Studies Library to support more partnerships.
To ensure that partnerships are transparent, transfers of value related to collaborative projects must also be disclosed via the Disclosure UK database (see Appendix 2 for further details on Disclosure UK, including how any contracted service values to patient organisations are disclosed).
Key resource: Recommended Summary of Project Outcomes Framework
Case study 3
View a case study demonstrating the step-by-step implementation of a project that involved system level partners.
MSD UK & Somerset, Wiltshire, Avon & Gloucestershire (SWAG) Cancer Alliance prehabilitation information platform project: collaborative working
The project objectives:
- Align SWAG regional healthcare professionals around standardised prehabilitation content and facilitate their involvement in the development of the digital platform.
- Reduce the variation in the quality of prehabilitation services across the region.
- Reduce health inequalities for underserved populations and who find healthcare services difficult to access.
- Patient engagement, education and activation may increase, enabling the patient to make more informed choices regarding their health.
- Improve patient’s ability to build mental and physical resilience’s during and beyond their cancer journey.
- Support and potentially improve patient health literacy.
- Improve patient experience.
How was the project identified?
- MSD has a field-based oncology healthcare team whose remit is to support and develop cancer services across cancer alliances, and to improve patient experience.
- This team regularly engages with system-wide, cancer alliance and trust stakeholders to understand their needs, to help support service redesign and pathway improvements.
- For this project, a discussion had initially been held with contacts built up over time at the South Central and West Commissioning Support Unit, around the importance of prehabilitation. This then led introductions to relevant teams within the cancer alliance.
- The project was then identified through discussions with the cancer alliance prehabilitation lead around the variation that exists in how prehabilitation services across the SWAG region are coordinated and commissioned.
- These discussions led to the idea to create a digital prehabilitation platform that each service could access, and which would provide a degree of consistency to each regional prehabilitation service.
- The cancer alliance requested MSD to help project manage the creation of the digital prehabilitation platform through the alignment of regional stakeholders and the facilitation of task and finish group meetings through which prehab content sourced and decided upon.
Guidance used
- ABPI Collaborative Working Project checklist.
- MSDs internal Collaborative Working How To Guide, which is an interactive toolkit to enable oncology healthcare to manage their projects in line with Clause 20 from the ABPI.
- Medical, legal and business practices cross-functional team as part of the governance and subject matter experts to ensure the project stays in line with internal ways of working as well as the code.
Risk management
- Issue and risk management plans were included from the outset in the project concept document with responsibility allocated across both organisations.
- A clear exit strategy was included in the project initiation document and collaborative working agreement stipulating that if either party was unhappy at any stage, the project could be terminated.
Stakeholders across all organisations – SWAG Cancer Alliance, trusts, and MSD were aligned within a task and finish group, terms of reference were drawn up to be clear of requirements from each party and ensure parity across the six trusts.
How was the project monitored and reported
- The project was monitored through:
- regular core project steering team meetings to assess progress
- regular task and finish group meetings with clear agendas
- regular sub-group meetings and engagement aligned to the three prehabilitation pillars of nutrition, exercise and wellbeing
- a project plan, which included objectives and clear assignment of responsibility for the tasks
- an action log was reviewed at each meeting to monitor progress
- a communication plan which detailed stakeholder organisations to engage with at the launch stage of the platform
- collaborative working end-of-project survey completion for NHS stakeholders, to share experience of working with MSD.
- Project executive summary and outcome summary written and published on the MSD corporate website.
Outputs
- The creation of a digital prehabilitation platform, built using feedback, insights, and recommendations from a multi-disciplinary team of healthcare professionals (HCPs) representing each of the trusts from across the region.
- Increasing access to and viewing of the platform as measured through the number of views on a weekly and monthly basis. From August 23 to December 23:
- monthly views increased from 140 to 222
- monthly users increased from 77 to 138.
- Provision of a prehabilitation resource for HCPs to access, irrespective of location or position, reducing variation in access to resources across the region
- Identification of prehabilitation as a regional priority that is now listed as such on the SWAG Cancer Alliance Webpage
- Dissemination of the Prehab Hub as a digital resource to a wide range of NHS stakeholder organisations, institutes, networks and charities through presentations and written communications both regionally and nationally, including:
- a case study written for the National Living With and Beyond Cancer team which has published the case study on its NHS Futures site and has also circulated the case study in its December 2023 newsletter
- a case study blog written for the Cancer Care Map team and posted on its web page
- a poster containing prehab digital platform details to be circulated to relevant HCPs in the region.
Date of preparation: May 2024
Job code: GB-NON-09265
Further reading
- Collaborate to innovate: learning from NHS, charity and life sciences industry experience to build a culture of research and innovation in the UK (April 2024)
- Partnering with purpose: how integrated care systems and industry can work better together (November 2023)
- Transforming lives, improving health outcomes: tackling the true cost of variation in uptake of innovative medicines (January 2023)
- Working Together - A Guide for the NHS, Healthcare Organisations and Pharmaceutical Companies (April 2022)
- Joint Working - A Toolkit for Industry and the NHS (September 2019)
- Simplifying cross-sector working between NHS Integrated Care Systems, Sustainability and Transformation Partnerships and industry: Guidance on governance and process (May 2019)
- Joint Working – A Quick Start Reference Guide for NHS and Pharmaceutical Industry Partners (2012)
* Disclaimer: Please be aware that this publication replaces the previous guidance highlighted above. Any publications not already archived will be so once this report is published.
Editable resources
- Checklist for determining if a project is collaborative working, joint working or neither
- Project Concept Framework
- Project Initiation Document
- Collaborative/Joint Working Agreement Framework
- Collaborative / Joint Working Project Executive Summary Framework
- Project Amendment Framework
- Summary of project outcomes
Acknowledgements
On 5 March, 12 March and 27 March 2024, roundtables were held with industry and local NHS organisation leaders from across primary, secondary and system care settings.
We express our gratitude to all colleagues who dedicated their time and insights during the three roundtables, integral in shaping the development of this guidance.
Please be aware that the guidance in this paper, developed by NHS Confederation or ABPI, may not reflect the opinions of the mentioned organisations and individuals.
| Name | Role | Organisation |
|---|---|---|
| Jennifer Gilroy-Cheetham | Senior NHS Engagement Manager | Chiesi |
| John Byrne | Chief Medical Officer | South West London ICB |
| Kelly McCormick | NHS Engagement and Access Manager | Sanofi |
| Louise Organista | Advanced Clinical Pharmacist | University Hospitals of Derby and Burton NHS Foundation Trust |
| Michael Smith | Chief Executive | Bolton GP Federation |
| Mike Stansfield | Market Access Project Support Manager | Sanofi |
| Nabil Rastani | Strategic Partnership Policy Manager | ABPI |
| Rakesh Marwaha | Managing Partner and Chief Executive | Erewash PCN |
| Rebecca Gale | Assistant Director, Primary Care Network | NHS Confederation |
| Roisin McCann | Senior Industry Engagement and Policy Officer | NHS Confederation |
| Rory Deighton | Director, Acute Network | NHS Confederation |
| Ross Rotherham | Health System Engagement Lead | Boehringer Ingelheim |
| Sarah Ferry | Senior Policy Advisor, Innovation and Life Sciences | NHS Confederation |
| Sarah Walter | Director, ICS Network | NHS Confederation |
| Sarika Passi | National Oncology Projects Manager UK | MSD |
| Su Jones | Collaborative Working Owner | AstraZeneca |
| Sue Consterdine | Associate Director, NHS Partnership and Strategy Lead | Bristol Myers Squibb |
| Tracey Palmer | Oncology Business Manager | Lancashire Teaching Hospitals NHS Foundation Trust |
Appendices
Appendix 1: The ABPI Code of Practice
The 2024 ABPI Code of Practice exists to regulate the promotion of prescription medicines to UK health professionals, industry interactions with health professionals, and the provision of information about prescription-only medicines to the public, including patients and patient organisations.
It is administered by the Prescription Medicines Code of Practice Authority (PMCPA) and is the cornerstone of the UK system of industry self-regulation.
All NHS-industry partnerships are bound by the code, which serves as a guardrail by which industry is regulated to ensure that throughout all collaborations, patient safety is maintained, in a professional, ethical and transparent manner to ensure the appropriate provision of high quality care. At its heart, the code gives confidence to local NHS organisations that partnerships operate in a clear and robust framework.
Strong support is given to the code by the industry, with all companies devoting considerable resources to ensure that their activities comply with it. Any complaint made against a company under the code is regarded as a serious matter both by that company and by the industry as a whole. Sanctions are applied against a company ruled in breach of the code.
Underpinning this are the ABPI Principles, which sit alongside the code. These set out the behaviours that embody the spirit of the code, and the ABPI expects that companies build these into their culture and approach.
The four key principles are as follows:
- Commitment to benefiting patients and ensuring patient safety by operating in a professional, ethical and transparent manner to ensure the appropriate and rational use of medicines and to support the provision of high-quality healthcare.
- Acting with integrity and commit to engaging in relationships which are responsible, professional, ethical and transparent.
- Commitment to ensuring that transparency is respected.
- Interact with all stakeholders with respect.
The ABPI Code reflects and extends beyond relevant UK legislation and ensures that the ABPI meets its commitments to implement other codes, such as the International Federation of Pharmaceutical Manufacturers and Traders and European Federation of Pharmaceutical Industries and Associations Codes.
The code is also supplemented by Disclosure UK, a Europe-wide initiative to increase transparency between pharmaceutical companies and the organisations they work with. Further information on Disclosure UK can be found in Appendix 2.
Appendix 2: Disclosure UK
The relationship between the pharmaceutical industry healthcare professionals and healthcare organisations plays a vital role in the development of life-enhancing and life-saving medicines.
At the core of the relationship is sharing knowledge to improve patient outcomes. To ensure this relationship is open and transparent, the pharmaceutical industry has taken the lead on disclosing ‘transfers of value’ – payments and benefits-in-kind – made by industry to healthcare professionals and healthcare organisations through Disclosure UK, a publicly searchable database, hosted by the ABPI. Disclosure UK is part of a Europe-wide initiative to increase transparency between pharmaceutical companies and healthcare professionals and organisations.
Data shown on Disclosure UK covers certain key areas of cross-sector working between industry, healthcare professionals, other relevant decision makers, and healthcare organisations, including:
- participation in advisory boards
- speaking at or chairing meetings
- working with and advising doctors and scientists in pharmaceutical companies
- speaking at conferences and symposia
- attending and contributing to national and international conferences
- participating in medical education and training funded by pharmaceutical companies
- provision of grants and donations to healthcare organisations
- sponsorship of healthcare organisation events for the provision of medical education to healthcare professionals.
Details of collaborative and joint working projects with healthcare organisations, among other things, are disclosed individually on the database. Certain research and development transfers of value to healthcare professionals, other relevant decision makers, and healthcare organisations are also disclosed in aggregate.
Separately, pharmaceutical companies are also required to disclose transfers of value made to patient organisations (and fees for certain contracted services paid to members of the public, including patients and journalists). The ABPI Code requires that this information is published on the pharmaceutical companies’ websites and that there are gateway links to this information from Disclosure UK.
For more resources and to search the database, please visit www.disclosureuk.org.uk.



